|
What Is Air Pressure, And What Links it To the History of the Barometer?Most Of Us Know That The Barometer Measures Air Pressure, But Which Was Thought Of First?What is Air Pressure? Air pressure is the effect of the weight of air surrounding the earth, expressed as so much weight per unit of area - 14.7 poundsper square inch for example. It's a pretty subtle sort of thing too. If we have 14.7 lbs pressing down on every square inch of us, why don't we feel weighed down? Three reasons - first it's equally distributedall over us, it's much less than the force of gravity, whichwe do feel, and we also don't feel crushed because the pressureinside our lungs is the same as the air outside, which we breathed in. Not so obvious, and that's probably why it took so long to discover. So let's consider what led to the discovery of air pressure, before checking out some air pressure facts and figures. Air Pressure and the Early History of the Barometer The development of the concept of air pressure is a classicexample of how scientific thought and experiment can haveresults that go far beyond the original problem.
In the early 1600s, engineers found that no matter what they did, their piston type pumps would not lift water more than about 30 feet in one cycle. Galileo was consulted, and though he didn't solve the problem he realised that a partial vacuum was created during the action of the pump Galileo's studies were followed up by Gasparo Berti in 1640. He filled a glass topped lead pipe over 35 feet long (10.7m) and attached it to the side of his house. After submerging the base of the pipe in an open container of water, he opened a valve. Water flowed down into the container, leaving a vacuum at the top. The column of water that remained in the pipe was a little over 30 feet high, a little more than the engineers managed in their less than perfect pumps. That was fine, but the experiment was a little unwieldy - neighbours were talking.
He was successful, as was my science teacher when he repeated the procedure in front of a group of appreciative 12 year olds not quite so many years ago. Torricelli deduced that something must be pushing the mercury and water up their respective tubes. He found that that the height of the liquid column varied in exactly the same ratio as the differences in density between mercury and water. It was also apparent that the diameter of the tube had no effect on the height reached by the mercury. This outside force could only be the weight of a mass of air acting on the open basin at the bottom of the tube - in other words air pressure. How Can We Demonstrate Air Pressure? A convincing demonstration of this was also part of my first science lesson. Here's how it goes. Take a small rectangular tin or can which can be sealed by a screw-on top. Put a small amount of water in the bottom and heat until the water is boiling and steam can be seen coming out of the opening. Take the tin away from theheat, and with great care, and preferably a pair of gloves, screw on the lid. As the tin cools down, it will gradually buckle and collapse, making some very satisfactory noises as it slowly folds in on itself. Very impressive for new science students, but what has happened here? Once the water started boiling, liquid water turned into water vapor, which is invisible until it cools down and we can see steam. The water vapor drives all the air out of the tin, so that when the lid is put on, the tin contains nothing but water vapor. As the tin cools, the vapor turns back to liquid water, slowly creating a partial vacuum, and a much lower pressure inside the tin. Before the water was boiled, the air in the tin was at the same pressure as the air outside, but now it has gone. There is nothing to act against the pressure of the outside air, and the tin slowly collapses. This experiment doesn't work as well with stronger cylindrical tins, and must never be attempted with a glass container. Air Pressure Standards - A Short Deviation Just so you have something to hang your hat on, the standard air pressure at sea level, accepted by all the world's meteorological organizations, is 1013mb at a temperature of 15°C. Although I give both metric and imperial values for most things in these articles, the metric system is standard throughout science, including meteorology. Equivalent values are 14.7 lbs/sq in, 29.92 inches or 76cm or 760mm of mercury, or 101.3 hectopascals, at a temperature of 59°F. It's best not to mix the two systems, so use °F with pounds/square inch and inches of mercury, and °C with everything else. Some of you may be wondering why the metric system uses millibars rather than, say, grams/sq cm. Good question, but the answer is confused by different concepts of weight, mass and pressure, and is far too complex to cover here. However we can say that a millibar is equivalent to the pressure exerted by a kiligram on a square centimetre, and that it is a word honored by a long history and familiar to all meteorologists. Let's move on quickly. Air pressure decreases with height at about 1" of mercury per 1000', or by about 38 millibars over the same height. Metrically, this transforms to about 1 millibar for every 8.2 metre change in elevation. This simple conversion only applies over the first 3000 feet or 1000m above sea level, after which pressure continues to decrease at less consistent rate. This decrease in air pressure with height is very significant to us. It explains why climbers need oxygen at altitude - the pressure at the top of Mount Everest is about one hundredth that at sea level. Planes fly at that height too - 30,000 feet or more - and their strong construction, thick small windows and circular cross section are all designed to maintain a breathable atmosphere for travellers, at a much greater air pressure than that outside. That's also why oxygen becomes immediately available if the plane loses inside air pressure. So after that small diversion into the numbers of air pressure its ...... Back to Torricelli, and The History of the Barometer
Over time, Torricelli observed that the level of mercury in the tube varied a little from day to day, suggesting that air pressure varied, and it wasn't such a big step to relate this to changes in the weather. And so the barometer was born, although it wasn't until later that it was given that name. It also wasn't immediately apparent why air pressure changes were so significant. Probably since the first time mankind learnt to grow food, a the major concern has been the weather,and how to predict it. It probably didn't matter so much in the earliest settled areas of the Middle East, where crops were irrigated from major rivers such as the Nile and Tigris-Euphrates, but in more remote areas, and on the coast where fishing and the beginnings of trade were important, the prediction of future weather changes was very important. Our ancestors, wherever they were , were not totally without resources. Astronomy developed early in most societies and was important in predicting seasonal changes, and you can bet that the weather typical of each season was well remembered. Among these were seasonal winds, and the weather they brought with them. We know that weathervanes were invented early - the first one known was at the Tower of the Winds in Athens around 400 BC. But apart from watching the clouds and looking out for changes in wind direction, it was around 2000 years before the next major advance in weather recording and forecasting. And that was the growing realization that changes in air pressure accompanied or foretold changes in weather. Most of us are familiar with the highs and lows that are talked about by TV weather people and shown on the weather maps. These are areas of relatively high and low pressure, and it is their movement that brings our ever changing weather. Just how significant changes in air pressure became more obvious as the usefulness of the barometer became apparent. And what of the answer to the question at the top of the page? Well, it seems that the principle of the mercury barometer came before the concept of air pressure was developed. But the naming, and the development of the barometer as an instrument to measure air pressure, had to wait another 20 or so years, as we shall see in the next article on the History of the Barometer. And just in case you were wondering, here is a page on How To Use A Barometer. It's not as easy as you might think to use a home barometer for weather forecasting This link will take you back to the Top, or, when you're ready, here's how to return to the Home page.
You may be interested to know that you can find out more about weather and home weather stations by receiving our newsletter ,"Watching Weather". It's published more or less weekly, and apart from tips on how to use your weather station and understand what it's telling you about the weather around you, it also covers many other weather related topics. If this sounds interesting, just add your name and email address to the form below. When you join, you'll also receive, totally free, a 20 page guide to setting up and trouble shooting problems in home weather stations. And I promise that you won't get spammed, and that your sign up details will remain totally confidential. Sign up now and receive your first issue almost immediately. Last update 05/28/2011
|





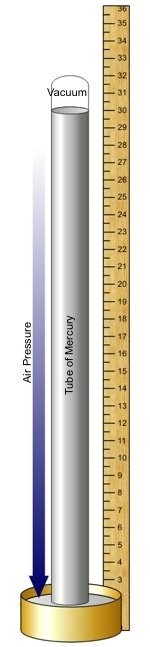 In 1643, Evangelista Torricelli, reputedly following up a suggestion from the now dead Galileo, tried the same experiment with mercury, a liquid 13.59 times heavier than water. Again his aim was to create a vacuum, but with mercury he only needed a tube greater than 30 inches (76cm) high.
In 1643, Evangelista Torricelli, reputedly following up a suggestion from the now dead Galileo, tried the same experiment with mercury, a liquid 13.59 times heavier than water. Again his aim was to create a vacuum, but with mercury he only needed a tube greater than 30 inches (76cm) high.

